Awe-Inspiring Examples Of Info About Is It Easier To Remove An Electron From 2s Or 2p

The Great Electron Eviction
1. Why Location, Location, Location Matters for Electrons
So, you're pondering the cosmic real estate market, specifically which electron is easier to evict from its atomic abode: one chilling in the 2s orbital or one hanging out in the 2p orbital. It's a valid question, and the answer reveals some fascinating insights into the quirky world of quantum mechanics. Think of it like this: are you more likely to easily convince someone to move from a cozy apartment close to everything (the nucleus) or a slightly more spacious but less conveniently located house?
At the heart of this electron eviction process is something called ionization energy. Ionization energy, in layman's terms, is the amount of oomph (energy) needed to yank an electron completely away from an atom. The lower the ionization energy, the easier it is to remove the electron. Several factors influence this energy, including the electron's distance from the nucleus and the shielding effect of inner electrons.
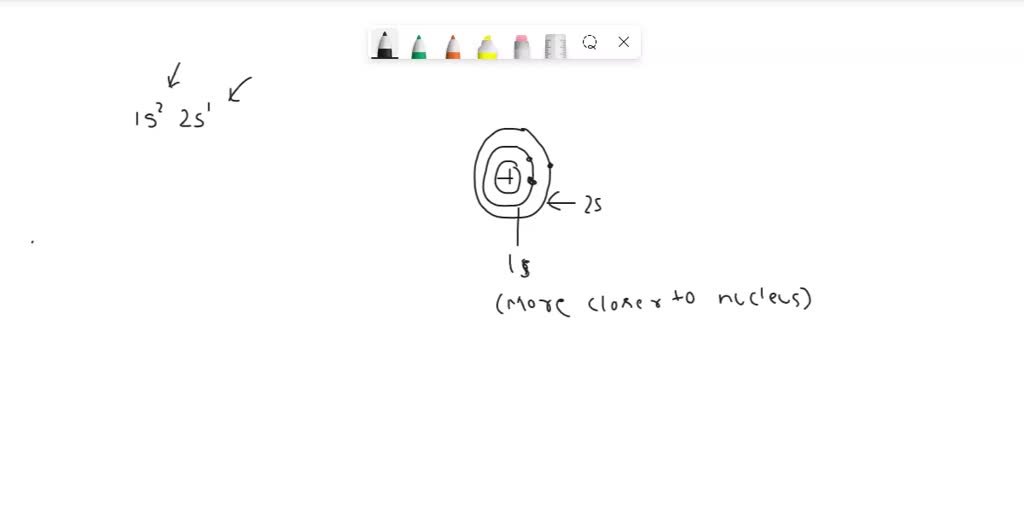
Now, both 2s and 2p electrons reside in the second energy level of an atom, meaning they're generally the same "distance" from the nucleus in terms of energy level. However, here's where things get a little more interesting. The "s" orbital is spherical, which means a 2s electron spends more time, on average, closer to the nucleus compared to a 2p electron, which occupies dumbbell-shaped orbitals oriented along different axes (x, y, and z).
Imagine the nucleus is a super attractive celebrity. The 2s electron is like a devoted fan constantly trying to get as close as possible. The 2p electrons are still fans, but they're a bit more spread out and have other things vying for their attention. It's harder to pull the devoted fan away from the celebrity, right?

The Shielding Saga
2. How Crowd Control Impacts Electron Removal
It's not just about proximity, though! There's also the issue of "shielding." Shielding refers to the effect of inner electrons blocking the full attractive force of the nucleus from reaching the outer electrons. Think of it as having a bunch of bodyguards (inner electrons) protecting the celebrity (nucleus) from adoring fans (outer electrons).
The 2s electron, because it spends more time near the nucleus, experiences less shielding than the 2p electron. Why? Because the 2s electron can effectively "penetrate" the shielding cloud created by the 1s electrons better than a 2p electron. This means the 2s electron feels a stronger effective nuclear charge, making it harder to remove. It's like the devoted fan briefly manages to slip past the bodyguards for a quick handshake, strengthening their bond with the celebrity.
To clarify, both 2s and 2p electrons do experience shielding. But the crucial point is that the 2s electron experiences less shielding. This seemingly small difference has a significant impact on the ionization energy.
Consider this analogy: imagine trying to pull someone away from a stage. If there's a thick crowd of people (shielding electrons) blocking your path, it's tougher. But if you can wiggle your way a little closer to the stage, partially bypassing the crowd, you'll have a harder time being pulled away. The 2s electron is wiggling closer!
SOLVED Why Is It Easier To Remove A 2s Electron From Li Compared
Ionization Energy
3. The Verdict
So, we've established that 2s electrons generally experience a stronger effective nuclear charge due to better penetration and less shielding. This boils down to the key takeaway: it's easier to remove a 2p electron than a 2s electron. The ionization energy for a 2p electron is lower than that for a 2s electron within the same energy level.
Think about it as a scientific tug-of-war. The nucleus is pulling the electrons in, and you're trying to pull them out. The electron that's held more tightly (the 2s electron) requires more force (energy) to be removed. The 2p electron, feeling a weaker pull from the nucleus, is easier to yank away.
This isn't just a theoretical curiosity. This principle is absolutely fundamental to understanding the chemical properties of elements. The ease with which an atom can lose an electron (or gain one, for that matter) dictates how it interacts with other atoms, forming molecules and driving all sorts of chemical reactions. Without understanding the nuances of electron behavior in different orbitals, chemistry would be a very confusing field!
This difference in ionization energy explains why certain elements are more likely to form certain types of bonds. Elements with valence electrons in p orbitals are generally more reactive than those with only s electrons in their valence shell, because the p electrons are more easily removed and thus more easily involved in bonding.

ELECTRON CONFIGURATIONS Ppt Download
Real-World Relevance
4. From Fireworks to Pharmaceuticals
Okay, so removing electrons might sound like a purely academic exercise, but its anything but. The ease (or difficulty) of removing electrons directly impacts the properties of materials around us. Think of fireworks — the vibrant colors are created by exciting electrons in various elements. When these excited electrons return to their ground state, they release energy in the form of light. The specific wavelengths of light emitted depend on the energy levels of the electrons, which are in turn influenced by factors like shielding and penetration, just like we've been discussing.
Beyond fireworks, understanding electron behavior is vital in designing everything from semiconductors in your phone to the catalysts that speed up chemical reactions in manufacturing. In the pharmaceutical industry, it's essential to understand how molecules interact with each other, which is driven by the distribution and behavior of electrons.
Consider solar panels. They work by using light to excite electrons in a semiconductor material, causing them to flow and create an electric current. The efficiency of a solar panel depends heavily on the material's ability to absorb light and release electrons easily. This, again, is tied directly to ionization energies and electron configuration.
So, the next time you see a dazzling firework display or marvel at the sleekness of your smartphone, remember the humble electron and the complex interplay of forces that govern its behavior. And remember that while it might seem easier to remove a 2p electron, both 2s and 2p electrons play crucial roles in shaping the world around us.

1) B Quartz Is The Only Covalent Network Solid Present Ppt Download
FAQ
5. Your Burning Questions Answered!
Still scratching your head? Here are a few common questions about electron removal and ionization energy:
6. Q
A: Generally, yes! 1s electrons are the closest to the nucleus and experience the strongest attractive force. They also experience minimal shielding. However, there can be exceptions in very heavy elements where relativistic effects come into play, but for most common elements, 1s electrons are the toughest customers to evict.
7. Q
A: That's a great question! Removing the first electron (the first ionization energy) is usually easier than removing the second, and so on. Why? Because when you remove an electron, you create a positive ion. This positive ion has a stronger pull on the remaining electrons, making them harder to remove. Imagine trying to pull a toy away from one child versus trying to pull it away from a group of increasingly protective children!
8. Q
A: Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. It's related to ionization energy and electron affinity (the energy change when an electron is added to an atom). Elements with high electronegativity tend to have high ionization energies and high electron affinities, meaning they strongly attract electrons and resist having them removed. Think of highly electronegative elements as electron hoarders!
