Awesome Tips About What Is The 10 Law Of Energy Flow

Unlocking the Secrets of Energy Flow
1. The Quest for a Complete Energy Picture
Ever wondered how energy zips around our world? We're taught about the basic laws of thermodynamics in school, but what about this intriguing "10th law of energy flow"? Before we dive in, let's clarify something crucial: there isn't a universally recognized "10th law of energy flow" in the same way we have the first and second laws of thermodynamics. These foundational laws are cornerstones of physics, well-established and widely accepted. However, the concept of a "10th law" often emerges when we try to understand more nuanced aspects of energy transfer, especially in complex systems like living organisms or ecological communities. Think of it as an ongoing quest to refine our understanding, rather than a brand-new, set-in-stone rule.
So, why the persistent interest in this "10th law"? It probably stems from a desire to have a more holistic view. The existing laws, while fundamental, sometimes seem insufficient to explain certain observed phenomena. For instance, the efficiency of energy transfer in ecosystems is often far lower than what traditional thermodynamic principles might predict. This sparks the question: are there other underlying principles at play that we haven't fully grasped yet?
One popular interpretation considers emergent properties in complex systems. When energy flows through a network of interacting components, the system as a whole can exhibit behaviors that are not simply the sum of its parts. This emergence could be seen as a "law-like" tendency for energy to organize itself in specific ways, creating structures and patterns that enhance the overall stability and function of the system. Imagine a flock of birds: each bird follows simple rules, but the collective behavior of the flock is incredibly complex and adaptive. Could something similar be happening with energy flow on a grander scale?
Ultimately, the "10th law of energy flow" is more of a metaphor than a formal scientific law. It represents the continuous effort to deepen our understanding of energy dynamics, particularly in situations where traditional laws alone don't provide a complete picture. It encourages us to explore new perspectives and to consider the intricate interplay of factors that influence how energy shapes the world around us.
How Does Matter Flow Through An Ecosystem Vrogue.co
What the Established Laws Already Tell Us
2. The Foundation We Build Upon
Okay, before we get too carried away with hypothetical laws, let's take a moment to appreciate the energy laws we do have. The first law, the law of conservation of energy, is a biggie: energy can't be created or destroyed, only transformed. It's like the ultimate energy budget, ensuring that everything balances out. Energy might change form — from potential to kinetic, or chemical to electrical — but the total amount remains the same. Think of it as the universe's way of saying, "No free lunch!"
Then we have the second law of thermodynamics, which introduces the concept of entropy. Entropy, in a nutshell, is a measure of disorder or randomness in a system. The second law states that in any energy transfer, the total entropy of an isolated system tends to increase over time. What does this mean in practice? It means that not all energy transformations are perfectly efficient. Some energy is always lost as heat, which is a less usable form of energy.
This "loss" isn't really a loss in the sense that the energy vanishes. It's more like a conversion to a form that's less available for doing work. Imagine a car engine: it converts chemical energy from gasoline into mechanical energy to move the car. However, a significant portion of the energy is also released as heat, which dissipates into the environment. This heat is still energy, but it's not directly contributing to the car's motion.
These two laws, taken together, paint a picture of a universe where energy is constantly flowing and changing, but always conserved. They provide a fundamental framework for understanding everything from the workings of a nuclear power plant to the metabolic processes within our own bodies. They are the bedrock upon which any discussion of a "10th law" must be built. Without a solid understanding of these principles, any attempt to define a new law would be, well, a little bit like trying to build a house on sand.

Energy Flows In The Ecosphere Ppt Download
Energy Flow in Biological Systems
3. Ecosystems and Efficiency
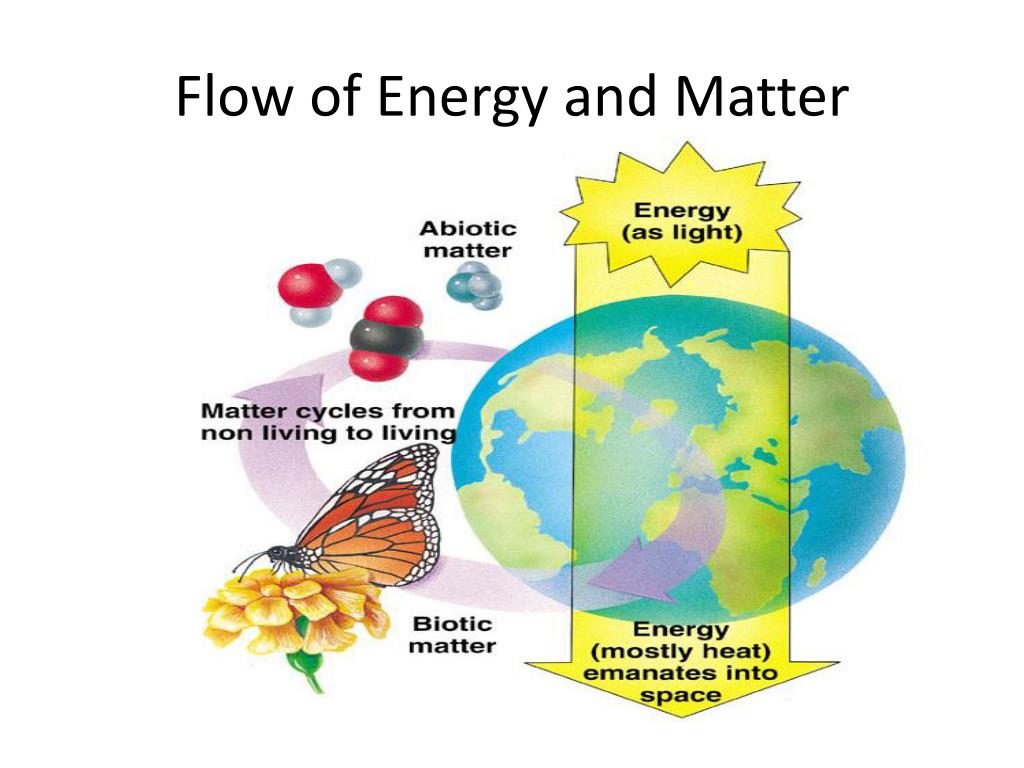
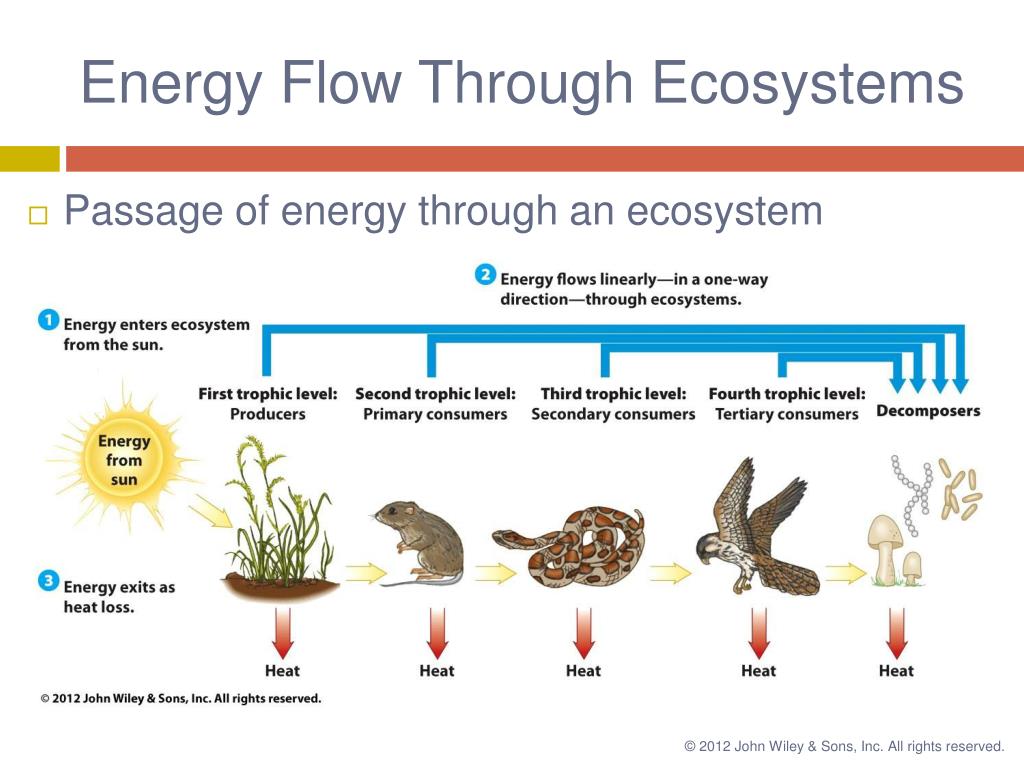
Now, let's move from the general principles of thermodynamics to the more specific context of biological systems. Here, things start to get a little more complicated. Energy flows through ecosystems in a hierarchical manner, from producers (like plants) to consumers (like animals). Each transfer of energy between trophic levels is accompanied by a significant loss of energy, primarily as heat due to metabolic processes.
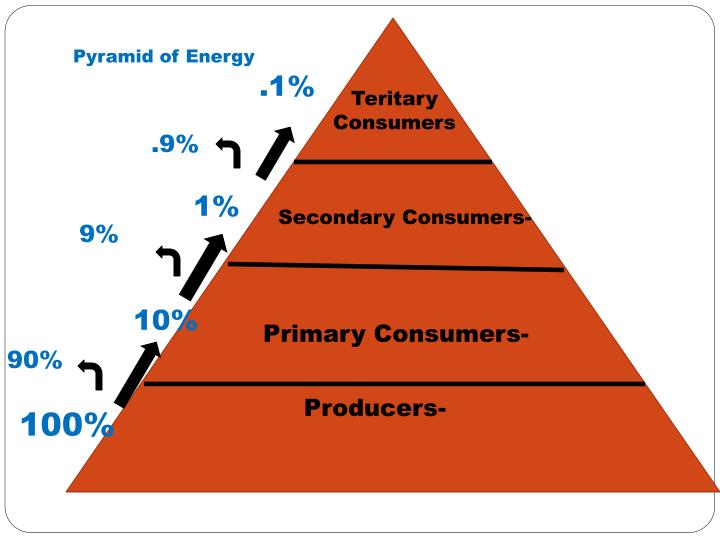
This loss of energy explains why food chains typically have only a few trophic levels. The amount of energy available at each successive level decreases significantly, making it impossible to support a large number of organisms at the higher levels. Think of it as a pyramid: the base, representing the producers, is wide and supports a large biomass. As you move up the pyramid to the higher trophic levels, the biomass decreases substantially.
But here's where the "10th law" idea might start to creep in. The efficiency of energy transfer between trophic levels is often surprisingly low. The "10% rule" is a common guideline, suggesting that only about 10% of the energy stored in one trophic level is converted into biomass in the next trophic level. The other 90% is lost as heat, used for respiration, or excreted as waste.
While the 10% rule is a useful simplification, it's important to remember that actual transfer efficiencies can vary widely depending on the specific ecosystem and the organisms involved. Some ecosystems, like those with highly efficient decomposers, may exhibit higher transfer efficiencies. Factors like age, size, and metabolic rate of the organisms also play a role. This variability is where the potential for a "10th law" — something that governs these efficiencies in more detail — feels compelling.

What Is The 10 Rule In Biology
Emergent Properties
4. Complexity and Energy Organization
One of the most intriguing aspects of complex systems is the phenomenon of emergent properties. These are properties that arise from the interactions of the individual components of the system, but that are not present in the components themselves. In other words, the whole is greater than the sum of its parts.
Consider a simple example: a bucket of water. The individual water molecules have certain properties, like mass and velocity. However, the bulk properties of water, like its boiling point or surface tension, are emergent properties that arise from the interactions between the water molecules. These properties are not simply the sum of the properties of the individual molecules.
In the context of energy flow, emergent properties can manifest in the form of self-organization. Energy can flow through a system in a way that creates patterns and structures that enhance the overall stability and function of the system. For example, in a forest, the flow of energy from sunlight to plants to animals creates a complex web of interactions that supports a diverse community of organisms.
Some researchers suggest that this self-organization of energy could be considered a "law-like" tendency, a principle that governs how energy shapes complex systems. This principle could potentially be incorporated into a broader understanding of energy flow, perhaps as a "10th law" that complements the existing laws of thermodynamics. It's a fascinating area of research with the potential to revolutionize our understanding of how energy drives the evolution and behavior of complex systems.
The Future of Energy Research
5. Exploring New Frontiers
So, while we don't have a definitive "10th law of energy flow" etched in stone, the ongoing pursuit of this concept highlights the ever-evolving nature of scientific inquiry. It encourages us to think beyond the established frameworks and to explore the more subtle and complex aspects of energy dynamics. This exploration involves delving into areas like network theory, information theory, and non-equilibrium thermodynamics.
Network theory, for example, can help us understand how energy flows through interconnected systems, revealing patterns and bottlenecks that might not be apparent from a purely linear perspective. Information theory provides a framework for quantifying the amount of information encoded in energy flows, which could shed light on how energy is used to create order and complexity.
Non-equilibrium thermodynamics extends the principles of thermodynamics to systems that are not in equilibrium, which is a more realistic representation of many natural systems. This branch of thermodynamics can help us understand how energy flows can create and maintain structures far from equilibrium, such as living organisms.
The quest for a "10th law" is, in essence, a call for a more holistic and integrated understanding of energy flow. It challenges us to move beyond reductionist approaches and to embrace the complexity and interconnectedness of the natural world. By exploring new frontiers in energy research, we can gain a deeper appreciation for the fundamental role that energy plays in shaping the universe and in sustaining life on Earth. Perhaps, someday, we will even discover a new principle that deserves to be recognized as the next great law of energy flow.
FAQs
6. Your Burning Questions Answered
Q: Is the "10th law of energy flow" a real, established scientific law?A: No, there isn't a formally recognized "10th law" in the same way we have the first and second laws of thermodynamics. The term often refers to the ongoing quest to understand more nuanced aspects of energy transfer in complex systems.
Q: What are the established laws of thermodynamics?A: The first law states that energy is conserved (it can't be created or destroyed, only transformed). The second law states that entropy (disorder) in an isolated system tends to increase over time, meaning energy transformations are never perfectly efficient, and some energy is always lost as heat.
Q: Why is energy transfer in ecosystems so inefficient?A: A significant portion of energy is lost as heat during metabolic processes at each trophic level. Organisms use energy for respiration, movement, and other activities, and some energy is excreted as waste. This inefficiency limits the length of food chains.